I agree my information will be processed in accordance with the ENSEMBLE³ Privacy Policy.
Sign up for our newsletter
I Privacy policy |
Functional Materials Technology Group
Optical Nanocharacterization Group
Inverse Materials Design Group
Next-Generation Energy Systems Group
Biophotonic Applications Group
Solar Energy Conversion Group
Oxide Single Crystals Group
A3B5 Compound Semiconductors Group
Functional Materials Laboratory
Oxide Single Crystals Laboratory
Materials Characterization Laboratory
III-V Compound Semiconductors Laboratory
Contact
Ensemble3 sp. z o.o.
01-919 Warsaw
133 Wólczyńska St.
NIP 1182211096
KRS 0000858669
Chemically Understanding the Liquid-Phase Synthesis of Argyrodite Solid Electrolyte Li6PS5Cl with the Highest Ionic Conductivity for All-Solid-State Batteries
📚🔋 Weekend is the perfect opportunity for some reading! 📖 So, we invite you to dive into our team's article available at the link: ARTICLE LINK
🔬🔋 "Chemically Understanding the Liquid-Phase Synthesis of Argyrodite Solid Electrolyte Li6PS5Cl with the Highest Ionic Conductivity for All-Solid-State Batteries" published in Chemistry of Materials.
Authors: Radian Febi Indrawan, Hirotada Gamo, Atsushi Nagai (Head of the Next-Generation Energy Systems Group at Ensemble3), and Atsunori Matsuda*.
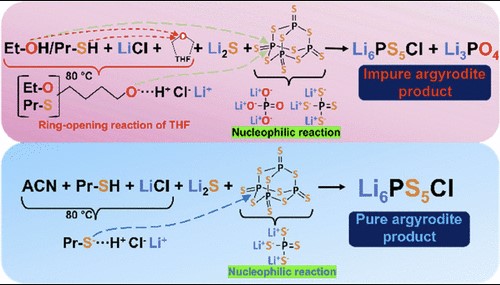
Solid electrolytes (SEs) are essential players acting as both electron separators and ion conductors in all-solid-state lithium-ion batteries. Liquid-phase synthesis shows great promise with its scalability and lower energy consumption. However, the complexity of SEs prepared this way leads to challenges, such as impurities, making liquid electrolytes indispensable.
This study addresses and resolves the question of why Li3PO4 impurity forms during the preparation of Li6PS5Cl argyrodite through liquid-phase synthesis. By using a thiol-based solvent instead of a hydroxide-based one, we successfully eliminated Li3PO4, resulting in Li6PS5Cl achieving the highest ionic conductivity value (>2 mS·cm–1) ever obtained through liquid-phase synthesis. Additionally, the absence of Li3PO4 in the argyrodite solid electrolyte significantly boosted the cell's capacity, providing remarkable stability.